Wash Chemistry and its Impact on Linen Life in your Commercial Laundry
During the wash process, laundry chemicals are introduced to aid in the removal of stains, oils, and other contaminants found in the goods. Under normal circumstances, the chemicals used in the wash process are properly dosed and adequately rinsed. If not managed properly, the chemicals can cause damage to the goods and shorten their service life. Here are some simple things to watch out for in your commercial laundry process that can reduce the lifespan of your textiles:
Proper sorting of the goods

Sorting goods in a commercial laundry operation
Failing to sort the laundry into classes (light soil, medium soil, severe soil, etc.) will have a detrimental affect on your operation. If lightly soiled goods are subjected to a heavy wash formula, the extended mechanical action and the higher dosage of detergents, bleach, and other chemicals will reduce the life expectancy of the textiles. Similarly, washing heavily soiled items with lightly soiled goods will simply ensure that these heavy soil items are washed over and over again, looping them through the laundry process as the same stains are found, then rewashed over and over. This inefficient loop will accelerate wear on the goods, remove them from normal service, and take up space in your process.
Overloading the washer-extractor
Overloading the washer with goods can mean that water and chemicals won’t be able to adequately reach all the goods, especially those goods in the center of the wash cylinder. Mechanical action will also be reduced, further decreasing the effectiveness of the wash. Finally, when these poorly laundered goods face the heat of the dryer or ironer, stains can be set. These goods must then be sent to a reclaim wash, which is much more aggressive than a standard wash further reducing their lifespan.
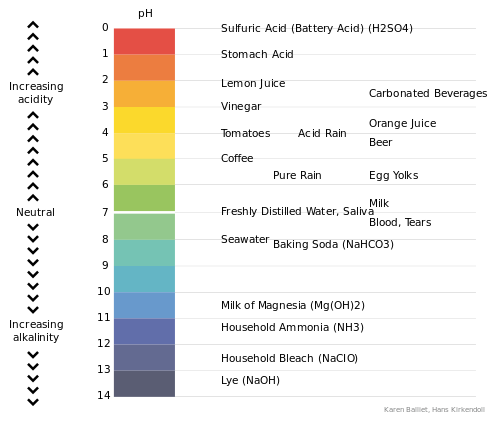
Improper pH levels after final rinse
If the pH level of the goods is not near neutral after washing, yellowing can occur. Bleach carry over, caused by too much bleach or not enough rinsing is a common problem, as synthetics like nylon or polyester can be damaged by over exposure, damaging the fibers and returning them to their natural color (yellow). Even natural fibers like cotton can suffer from this effect. High alkalinity can cause a reaction which forms dissolved salts in the wash solution. These salts can then be absorbed by the roll padding of the ironer, clogging it and resulting in poor moisture removal and an inefficient ironing process.
Although this is a simple overview, using these guidelines in your preventative maintenance program and your general laundry management will help to increase linen life and reduce consumption of water and wash chemicals, ultimately increasing your profits.